Low, Sustained Long-Term Results In Real-World, All-Comers Patients
Powerful Clinical Performance Sustained Out to 3 Years
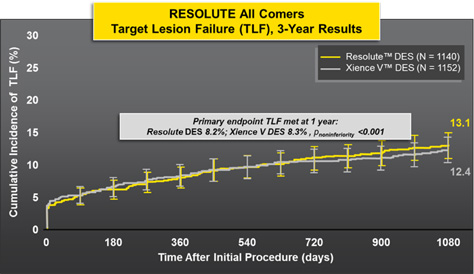
No Difference In Efficacy and Safety Endpoints at 3 Years
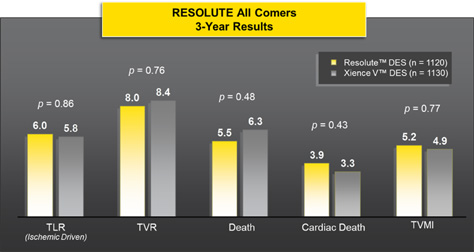
No Significant Difference in ARC Definite/Probable Stent Thrombosis at 3 Years
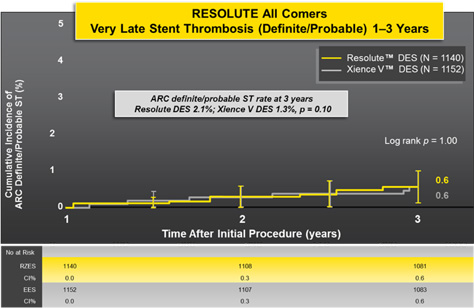
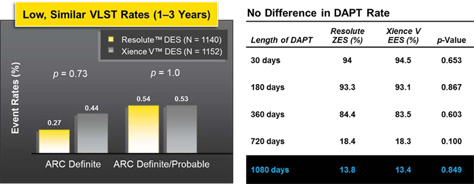
Low Rates of Very Late Stent Thrombosis (VLST) Despite Low DAPT Rate After 1 Year
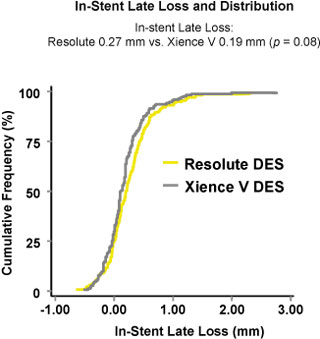
No Significant Difference in In-Stent Late Loss at 13-months
Target lesion failure = cardiac death, target vessel MI, TLR . RESOLUTE All Comers was not specifically designed or powered to compare endpoints other than TLF.
Error bars indicate a pointwise, two-sided 95% confidence interval (1.96 ± SD).
Standard error is based on the Greenwood formula.
All other p-values based on Fischer's Exact Test. p-values for outcome differences are uadjusted for multiple comparisons.
Innovative RESOLUTE All Comers Trial Design
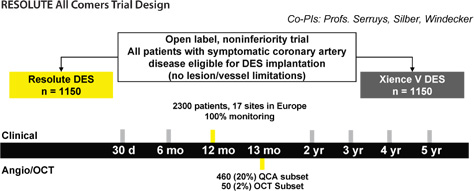
Primary endpoint: TLF (ARC-defined): Cardiac death, target vessel MI, clinically indicated TLR at 12 months.
Secondary endpoint (powered): % Diameter stenosis (in-stent) at 13 months.
Secondary endpoints: TLF at 30 d, 6 months, 2–5 yr; Composite (all death, all MI, any revascularisation) at each FU time point; angiographic and optical coherence tomography (OCT) parameters at 13 months
Drug therapy: ASA and clopidogrel/ticlid >6 months (per guidelines)
Patient Eligibility
Inclusion Criteria
Coronary artery disease
- Stable angina
- Silent ischemia
- Acute coronary syndrome including UA, NSTEMI and STEMI
Lesion characteristics
- Number of lesions: no limitation
- Number of vessels: no limitation
- Lesion length: no limitation
Exclusion Criteria
Known allergy to
Aspirin, clopidogrel, heparin, cobalt alloy, everolimus, zotarolimus, contrast material, polymer coating
Planned, elective surgery within 6 months of PCI
Unless dual anti-platelet therapy could be maintained
Pregnancy
Participation in another Trial
Patient baseline characteristics
| Resolute DES (N = 1140) |
Xience V DES (N = 1152) |
p-Value | |
| Age (yr) | 64.4 ±10.9 | 64.2 ±10.8 | NS |
| Men (%) | 76.7 | 77.2 | NS |
| Diabetes mellitus (%) | 23.5 | 23.4 | NS |
| ACS (%) | 48.3 | 47.7 | NS |
| AMI (within 12 hr) (%) | 15.4 | 17.8 | NS |
| AMI (within 72 hr) (%) | 28.9 | 28.8 | NS |
| Multivessel disease (%) | 58.4 | 59.2 | NS |
| Small vessel (RVD ≤2.75 mm) | 67.8 | 67.4 | NS |
| Long lesion (length >18 mm) | 18.2 | 21.2 | NS |
| In-stent restenosis (%) | 8.1 | 8.0 | NS |
| Bifurcation/ trifurcation | 16.9 | 17.7 | NS |
| Total occlusion (%) | 16.3 | 17.2 | NS |
| Complex Patients* (%) | 67.0 | 65.6 | NS |
*Complex patient definition: Bifurcation, bypass graft, ISR, AMI <72 hr, LVEF <30%, unprotected LM, >2 vessels stented, renal insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, >1 lesion per vessel, lesion with thrombus or TO (preprocedure TIMI = 0). With the exception of long lesions (treatable with a single 38-mm length stent), Resolute DES currently is not specifically approved for the patient subsets noted in this complex patient definition.
Lesion characteristics
| Resolute stent N = 1140 patients N = 1661 lesions |
Xience V DES N = 1152 patients N = 1705 lesions |
p-Value | |
| Lesions treated per patient | 1.46 ±0.73 | 1.48 ±0.77 | NS |
| Lesion Length (mm) | 11.89 ±7.50 | 12.15 ±7.86 | NS |
| No. of stents per patient | 1.9 ±1.2 | 2.0 ±1.3 | 0.02 |
| Stent length per patient (mm) | 34 ±24 | 37 ±26 | 0.02 |
| Pre-stent balloon dilatation | 69.5% | 70.2% | NS |
| Implantation of study stent | 98.0% | 96.9% | NS |
| Lesion success | 98.9% | 99.1% | NS |
| Device success | 97.0% | 96.6% | NS |
| Procedure success | 94.6% | 94.2% | NS |
