RESOLUTE US 36-Month Outcomes:
Low Event Rates of Stent Thrombosis
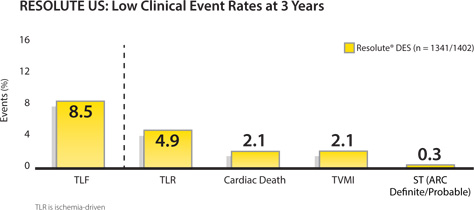
12 months primary endpoint of Target lesion failure (TLF) is defined as cardiac death, target vessel MI and TLR.
TLR is ischemia driven.
Excluding TLF, this study is not specifically designed or powered for the analysis shown.
Robust Trial Design That Enrolled a Broad Range of Patients and Lesions
Baseline Characteristics:
Broad range of patients with high percentage of challenging lesions
Patient baseline characteristics
Overall Cohort (2.25–4.00-mm diameter)
| Patient Characteristics | Resolute DES (n = 1402) |
| Age (yr) | 64.1 ±10.7 |
| Men (%) | 68 |
| Diabetes mellitus (%) | 34.4 |
| Insulin dependent (%) | 9.6 |
| Prior MI (%) | 21.6 |
| Prior PCI (%) | 32.7 |
| Prior CABG (%) | 8.8 |
| Mean ejection fraction (%) | 58.0 ±9.2 |
| Hyperlipidemia (%) | 87.7 |
| Hypertension (%) | 84.2 |
| Current smokers (%) | 20.9 |
| Stable angina (%) | 56.1 |
| Unstable angina (%) | 41.9 |
| MI (%) | 2.1 |
| Lesion Characteristics | Resolute DES (nL = 1573) |
| RVD (mm) | 2.59 ±0.47 |
| Minimal lumen diameter (mm) | 0.77 ±0.35 |
| Lesion length (mm) | 13.06 ±5.88 |
| Lesions treated per patient | 1.13 ±0.35 |
| Average DS (%) | 70.67 ±11.52 |
| Type B2/C lesion | 75.2 |
| Two vessels treated (%) | 10.4 |
| ≥1 small vessel (RVD ≤2.75 mm) (%) | 68.5 |
| ≥1 lesion length > 18 mm (%) | 20.0 |
| Vessel location | |
| LAD (%) | 45.9 |
| LCX (%) | 32.2 |
| RCA (%) | 31.2 |
| LMCA (%) | 0.6 |
