Strong New Data from RESOLUTE International Trial
Strength in Numbers
Low rates in all efficacy and safety endpoints
Large, high quality all-comers trial
- 2349 real-world all-comers patients enrolled from 88 international sites
- 100% independent clinical event adjudication
- High degree of monitoring
- 97.7% clinical follow-up at 12 months
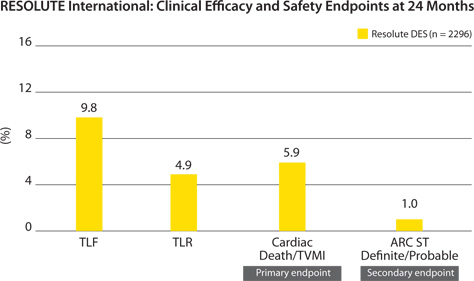
TLF = Cardiac death, target vessel MI, clinically indicated TLR.
Strength in Consistency
Consistent results to RESOLUTE All Comers
Large, high quality all-comers trial
- RESOLUTE International has similar minimal exclusion criteria to RESOLUTE All Comers
- Total of 3489 real-world Resolute DES patients enrolled in both all-comers trials
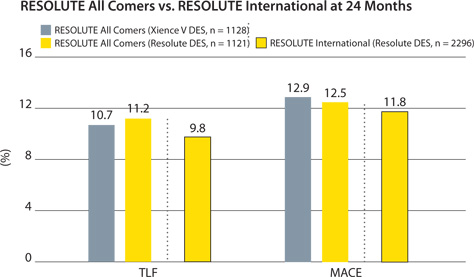
TLF = cardiac death, target vessel MI, clinically indicated TLR.
RESOLUTE International Trial Design
Large All Comer Trial
PI: J. Belardi, F–J. Neumann, P. Widimský
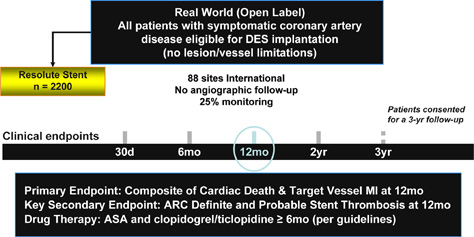
Patient Eligibility Similar to RESOLUTE All-Comers
Inclusion Criteria
Coronary artery disease
- Stable angina
- Silent ischemia
- Acute coronary syndrome including UA, NSTEMI and STEMI
Intention to electively implant at least one Resolute stent
Lesion characteristics
- Number of lesions: no limitation
- Number of vessels: no limitation
- Lesion length: no limitation
Written informed consent
Exclusion Criteria
Pregnancy
Inability to comply with follow-up requirements
Participation in another trial
Patient baseline characteristics
RESOLUTE International |
RESOLUTE All Comers |
||
| Resolute DES (n = 2349) |
Resolute DES (n = 1140) |
Xience V DES (n = 1152) |
|
| Age (yr) | 63.5 ±11.2 | 64.4 ±10.9 | 64.2 ±10.8 |
| Men (%) | 77.8 | 76.7 | 77.2 |
| Diabetes mellitus (%) | 30.5 | 23.5 | 23.4 |
| Insulin Dependent (%) |
9 | 8.4 | 7.1 |
| Prior MI (%) | 27 | 28.9 | 30.4 |
| Unstable Angina (%) | 26.1 | 19.4 | 18.9 |
| AMI (within 12 hr) (%) | 9.7 | 15.4 | 17.8 |
| AMI (within 72 hr) (%) | 20 | 28.9 | 28.8 |
| Lesions treated per patient | 1.3 ±0.7 | 1.5 ±0.7 | 1.5 ±0.8 |
| Multi vessel treated (%) | 14.0 | 25 | 25 |
| Small vessel (RVD ≤2.75 mm) | 45.4 | 67.8 | 67.4 |
| Long lesion (length >18 mm) | 46.1 | 18.2 | 21.2 |
| In-stent restenosis (%) | 7.6 | 8.1 | 8.0 |
| Bifurcation/trifurcation (%) | 18.2 | 16.9 | 17.7 |
| Total occlusion (%) | 6.3 | 16.3 | 17.2 |
| Complex Patients1 (%) | 67.5 | 67.0 | 65.6 |
Complex patient definition: Bifurcation, bypass graft, ISR, AMI <72 hr, LVEF <30%, unprotected LM, >2 vessels stented, renal insufficiency or failure (creatinine >140 µmol/L), lesion length >27 mm, >1 lesion per vessel, lesion with thrombus or TO (preprocedure TIMI = 0).
With the exception of small vessels and long lesions (treatable with a single 38-mm length stent), Resolute DES currently is not specifically approved for the patient subgroups noted above.
